Falsified medicines are a health risk.
The Medicines Verification System protects patients from falsified medicines and safeguards patient safety.
To dispense or distribute medicines in Finland, pharmacies and pharmaceutical wholesalers must be connected to the Finnish Medicines Verification System. This system is integrated across the entire pharmaceutical supply chain and is designed to prevent falsified medicines from entering the legal market.
When you purchase medicines from a licensed (online) pharmacy, you can trust that the product has been verified for authenticity.
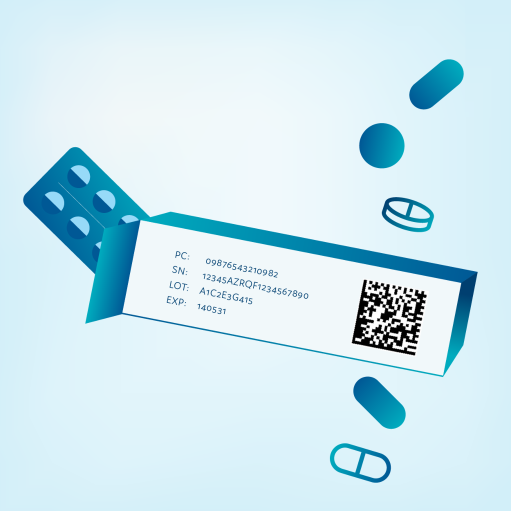
All prescription medicine packs must have safety features.
Pharmaceutical companies equip their packs with unique identifiers. The pack must also include features that reveal if the pack has been unlawfully opened (anti-tampering device, ATD). The unique information on the pack consists of the unique serial number and product code. This information can be found in the 2D code that is on the pack. In addition, the 2D code contains the batch number and the expiry date.
At the pharmacy, the 2D code is scanned and checked against the verification system. If the data doesn’t match, the system raises an alert. The pharmacist also checks that the ATD is intact. Only then can the medicine be safely dispensed to the patient.

The system is mandated by EU legislation, specifically the Falsified Medicines Directive, and a delegated regulation specifying it. The verification system primarily applies to prescription medicines, with some exceptions.
In Finland, the entire pharmaceutical supply chain participates in the system. Fimvo manages and develops the system, while Fimea, the Finnish Medicines Agency, supervises its operation.
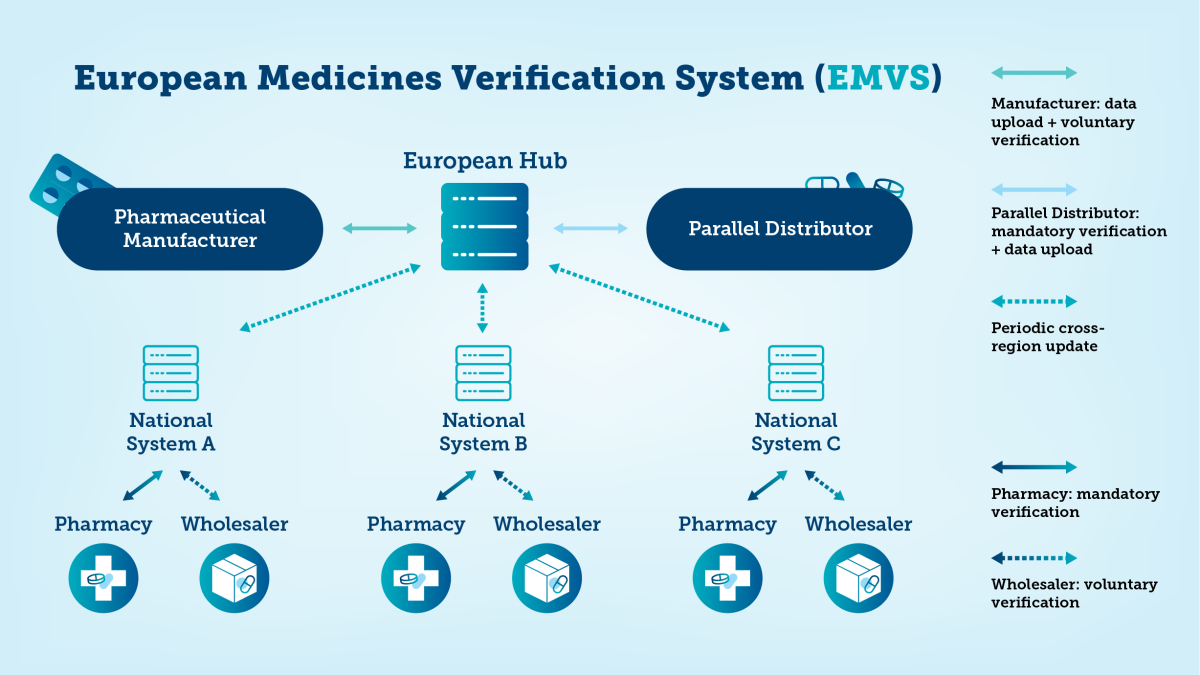
A Europe-wide network
Fimvo is part of a broader European system. The EU Hub is a central database where pharmaceutical manufacturers and parallel traders upload unique identifier data. Each EU and EEA country has its own national repository, which receives data from the EU Hub.
Local supply chain actors—wholesalers, pharmacies, hospital pharmacies, and private dispensaries—use these national systems. The EU Hub ensures cross-border safety for multi-market and parallel traded packs and supports efficient product recalls.
The national systems interact with each other via the EU Hub. This ensures the safety of multi-market packs. Also parallel traded packs can be correctly verified. In addition to this, a pan-European system is an efficient tool in case of a recall situation.
System Governance
The Medicines Verification System is governed by non-profit organisations, as required by EU law.
The European Medicines Verification Organisation (EMVO) oversees the EU Hub. EMVO’s members include representatives from the pharmaceutical industry, pharmacies, wholesalers, and hospital pharmacies.
A list of the all the national organisations i.e. NMVOs can be found on EMVO's website.
Data security
The system is designed with strict data protection rules:
- Data belongs solely to the entity that created it.
- Supply chain actors cannot access each other’s data.
- No personal data is stored in the system.
For more details, visit: European Commission's site on falsified medicines